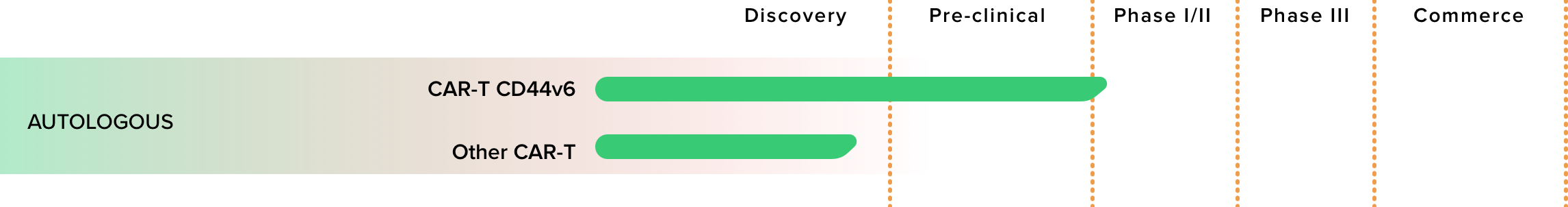
CAR-T CD44v6
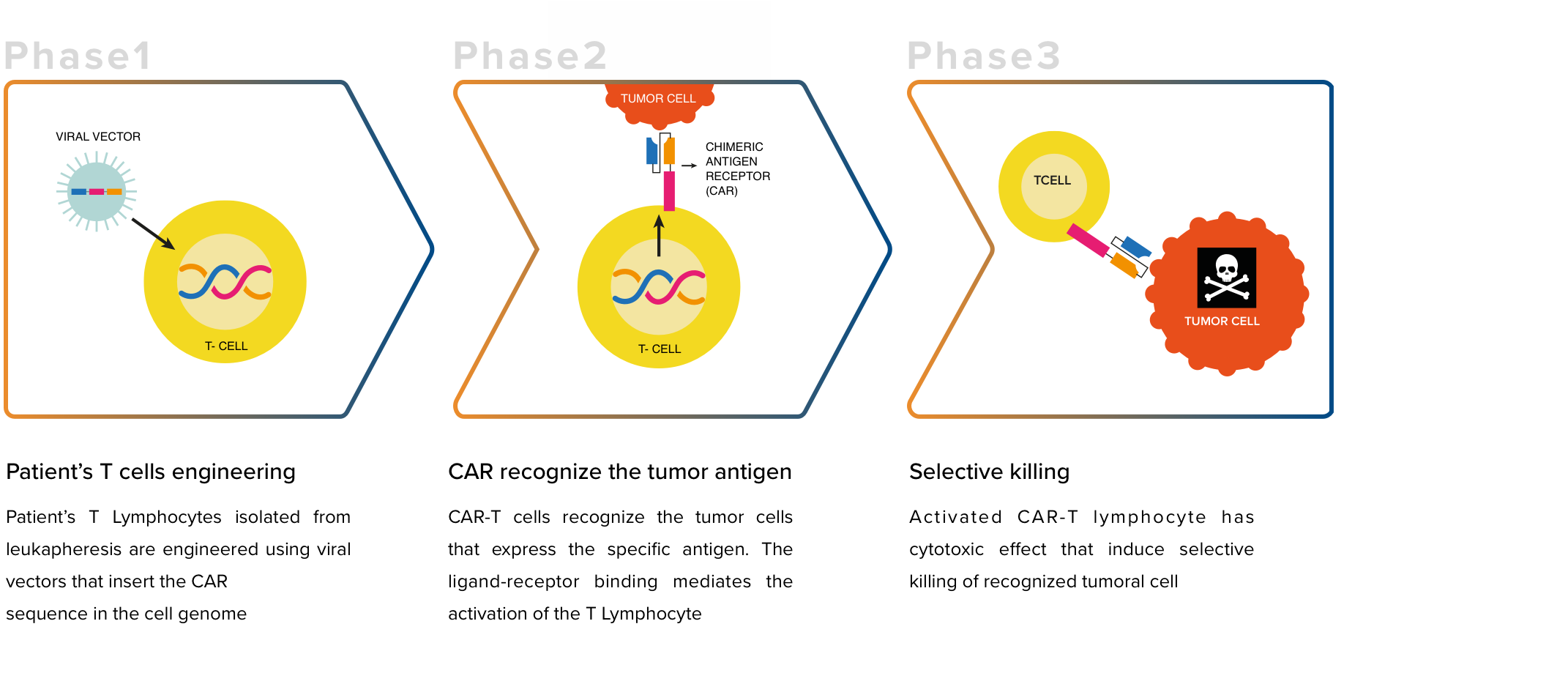
Chimeric receptors for the antigen (CAR), today represent a relevant therapeutic strategy in oncology and consist in the engineering of T lymphocytes with receptors directed against specific tumor antigens. The goal is to obtain T lymphocytes with the ability to selectively eliminate tumors, without attacking the
patient's healthy tissues.
CAR CD44v6 has a wide therapeutic potential as it is based on the recognition of variant 6 (v6) of the CD44 antigen (CD44v6) which is expressed by many haematological neoplasms (acute myeloid leukemia, multiple myeloma) and by numerous solid tumors of epithelial origin. (breast, lung, colon, pancreas, and head / neck).
The therapy with CAR-T CD44v6, involves isolating the patient&'s T cells and modifying them ex vivo with a viral vector. T cells are engineered to express the CAR and the HSV-TK suicide gene already used in Zalmoxis®. Thanks to the presence of CAR the lymphocytes recognize and kill the tumor cells, while the suicide gene allows to eliminate the T lymphocytes in case of toxic reaction against the patient's healthy tissues.
Once engineered, T cells expressing CAR are expanded in vitro until the required therapeutic dose is obtained and then infused into the patient. Before receiving the new cells, the patient undergoes a lymph- depleting chemotherapy, ie treatment with drugs that eliminate part of his T lymphocytes. This therapy favors the engraftment and permanence in the circulation of the T lymphocytes modified to express the CAR.
MolMed is also the coordinator of the EURE-CART project (EURopean Endeavor for Chimeric Antigen Receptor Therapies) which has obtained a European funding of 5,903,146 euros, as part of Horizon; aimed at demonstrating the safety and efficacy of immunotherapy based on CART-CD44v6 lymphocytes in acute myeloid leukemia and multiple myeloma.
Other CAR-Ts
Thanks to the experience gained in the development of autologous CD44v6 CAR-T, MolMed is developing autologous CAR-T products against different antigens selectively expressed by tumor cells.
The identification of the new tumor targets was based on a multidisciplinary approach coordinated by the members of the Scientific Advisory Board, in order to be potentially effective against a large number of neoplasms, both haematological and solid.
For the development of these products, MolMed will draw on one side of the experience acquired with the work done on the other proprietary products, on the other side MolMed has signed a Master Agreement with AbCheck sro, a subsidiary of Affimed GmbH and a European leader in identification and screening of antibodies.
On the basis of the agreement, AbCheck will use its proprietary platform for the research, selection, optimization and production of several human single-chain variable fragments (scFvs), able to specifically recognize each potential target chosen by MolMed. The scFvs are the portion of the CAR molecule dedicated to the specific recognition of the antigen expressed by the tumor cell.