MolMed offers development and production services thanks to its facilities in Milan and Bresso, suitable for the production and testing of viral vectors and genetically modified cells in accordance with GMP guidelines.
Both facilities have been designed applying the highest standards in the field of gene and cell therapy in terms of plants, finishes, and laboratory instrumentation
Facilities:
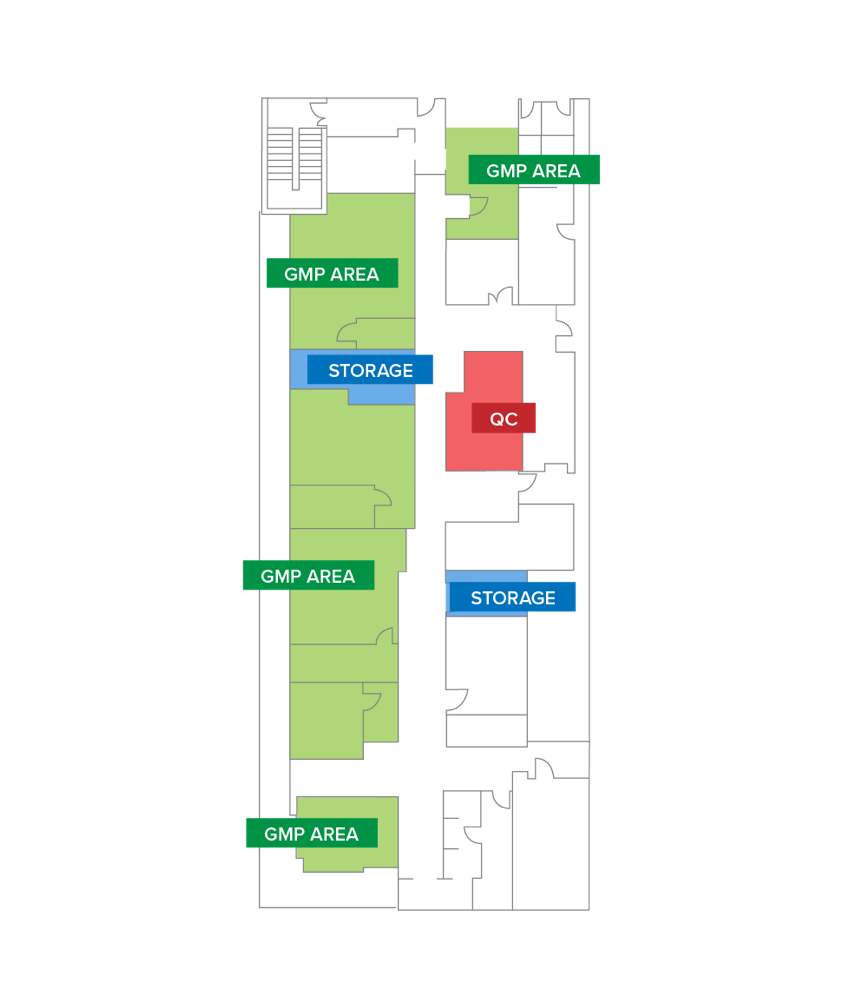
Milan Site_GMP
- Surface area: about 1.500 mq (16.000 SQF) dedicated to development, testing and production according to cGMP.
- Authorization to produce drugs for clinical and commercial use (2003 and 2015).
- More than 150 GMP batches of viral vectors and over 200 batches of genetically modified cells to support more than 20 clinical trials in Europe and the US
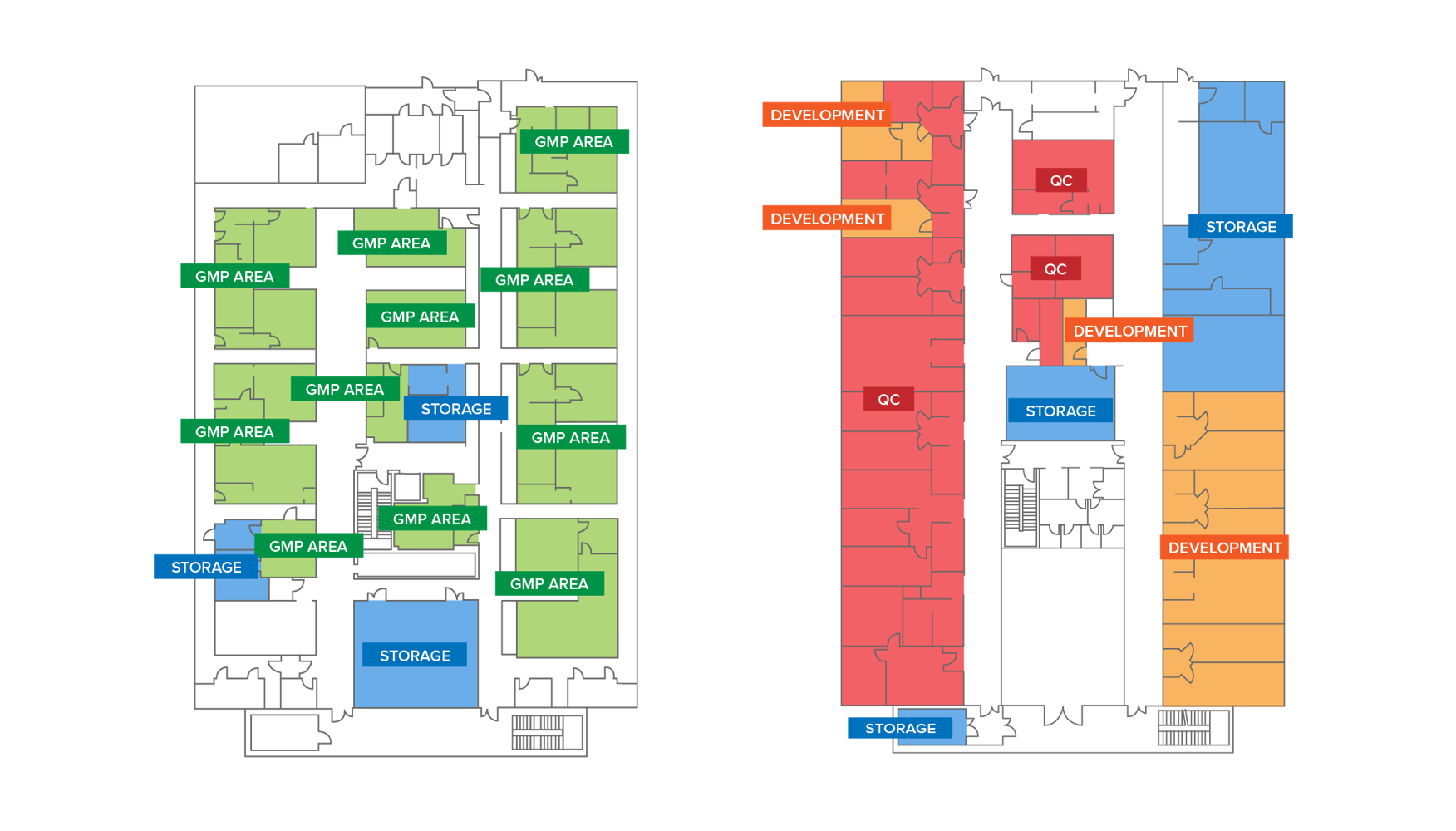
Bresso Site_GMP
-
Surface area: about 3.300mq (35.000 SQF) divided in two floors
-
Designed and built in accordance with the highest quality standards
-
First Floor: 1.600mq (17.000SQF) dedicated to development, testing and quality control
-
Second Floor: 1.700mq (18.000SQF) dedicated to the production of viral vectors and Genetically modified cells according to cGMP
-
10 Grade B suites and 7 Grade C suites
-
Authorization for the GMP production of viral vectors (on a 48L or 200L scale) and genetically modified cells for clinical trials and market