MolMed offers tailor-made services for cell and gene therapy projects, satisfying the customer's needs in terms of guaranteeing the highest clinical and commercial standards for ATMP.
Our services include technology transfer, development, scale up, automation and validation of RVV / LVV cell transduction processes, as well as the finalization and realization of the Medicinal Product. At the same time, MolMed offers the development, qualification and validation of analytical tests, both standard and product-specific. We have qualified, validated and internalized more than 100 analytical tests for the characterization and release of the products. MolMed is also able to assist its customers by offering proprietary processes for the cell manipulation of HSC (CD34 +) and T lymphocytes.
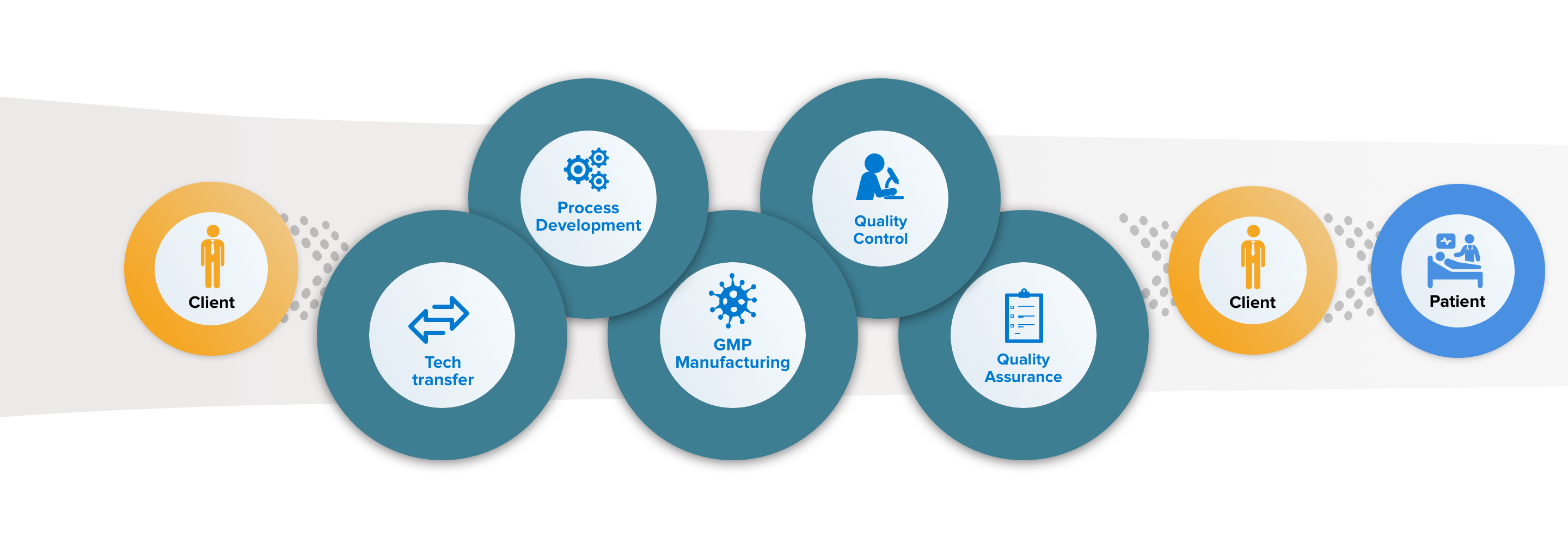
Technology Transfer
We offer proprietary process for CD34+ and T-cell transduction. In alternative, we can internalize and
further develop Client’s processes for clinical and commercial products.
Process Development and Analytical Methods Development
Our process development unit has performed several tech transfer, development, scale-up, automation
and validation activities for both proprietary and third parties’ transduction platforms.
GMP Manufacturing
We perform CD34+ and T cells transductions, for both autologous and allogeneic products.
Italian Health Authorities approved our two facilities for the production of both clinical and commercial
products.
Quality Control
We perform over 100 in-house tests including safety, identity, potency, purity on IPC and DS/DP, resulting in time and costs saving.
The release path is finalized by our internal QPs.
QA and Regulatory support
MolMed Quality management system is in line with EU ATMP GMP and Food and Drug Administration (FDA) guidelines.
We also offer support for Scientific Advice to national and international bodies, including documentation for Pre-IND, IND, IMPD and trial specific procedures, designation application (e.g. Orphan drug) and Filing for Marketing Authorization (and equivalent).




