With its long experience and extremely successful track record MolMed is one of the world leaders in GMP production of retroviral and lentiviral vectors.
We manufacture viral vectors in multiple scales, with the aim of satisfy Customer’s needs both for clinical and commercial use.
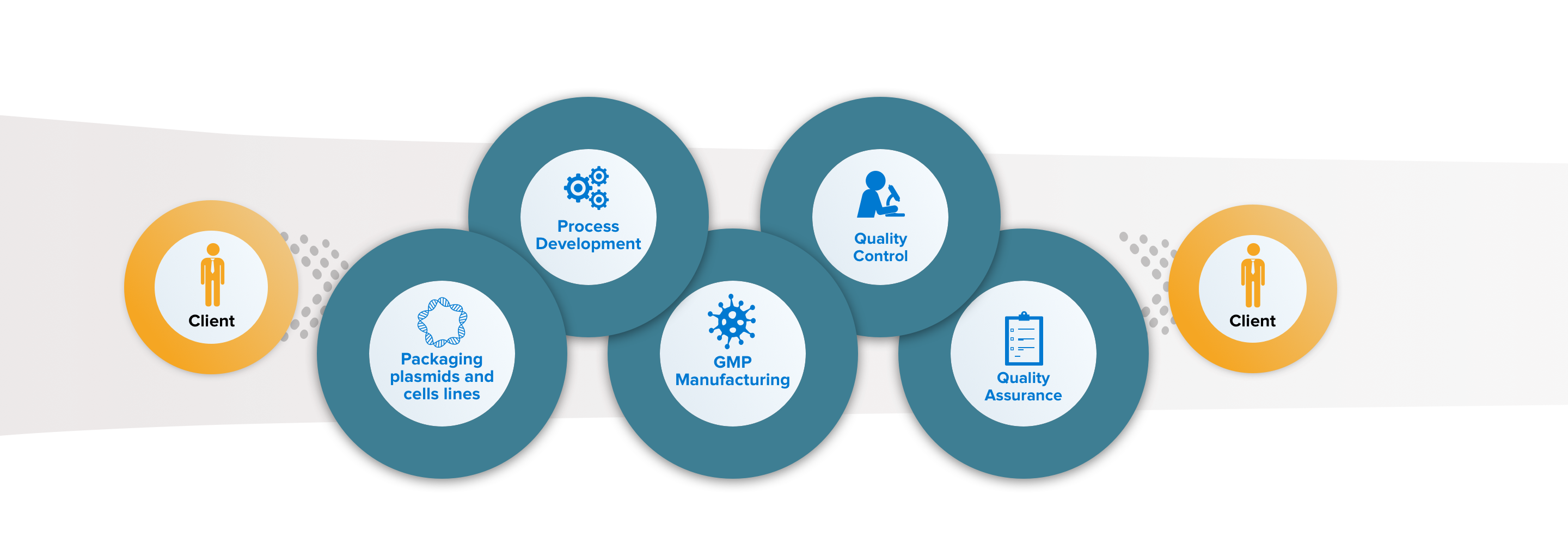
MolMed Proprietary Technology
MolMed offers proprietary packaging plasmids and producer cell lines for vector manufacturing.
Process Development and Analytical Methods Development
We internally perform process optimization, scale up and automation of viral vectors manufacturing processes. In parallel we develop, validate and qualify product-specific analytical methods.
GMP Manufacturing
We offer 48L and 200L batches for RVV and LVV manufacturing, based on our proprietary processes. In addition, we also produce GMP Master and Working Cell Bank (MCB, WCB) of different cell types.
Our facilities are approved by Italian Health Authorities for production of both clinical and commercial products.
QC Testing
We perform over 100 in-house tests for vectors including safety, potency, characterization and purity, resulting in time and costs saving.
QA and Regulatory support
MolMed Quality management system is in line with EU ATMP GMP and Food and Drug Administration (FDA) guidelines.
We also offer Client support for Scientific Advice to national and international bodies, including documentation for Pre-IND, IND, IMPD and trial specific procedures, designation application (e.g. Orphan drug) and Filing for Marketing Authorization (and equivalent)




